Quality Control
QUALITY CONTROL OF PERFECTION
Incoming Quality Control
Quality control department is a quality assurance within the normal range that extends for all products and services. It collects materials through order of the manufacturer, checks the specifications, quantity, and seals the confirmation according to the sampling standard of the incoming quality control, specification, all specified dimensions, appearance and characteristics records. If the inspection is not qualified, the applications of quality abnormality improvement notice and purchase return slip will be written to reduce product problems and safe storage.
Manufacturing and Inspection
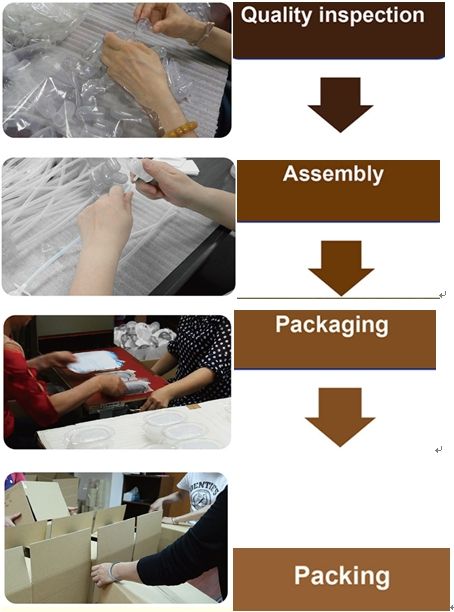
Before production and assembly, the production will be confirmed only after passing the quality control. During the operation, we will go through inspections and strictly check that the testing equipment is operating properly. Unqualified parts are determined by the quality control. The non-conforming product control will be returned, and the qualified product is stored in the warehouse.
Quality Analysis and Improvement
Remediation of non-conformity products for corrective and preventive measures in order to reduce product problems. We will re-qualify and review the unqualified products immediately that need to be scrapped, returned, corrected and prevented. Also, we recruit and quality control departments, auditors and the business department together to investigate, analyze, review and the final report will be approved by the general manager. Meantime, the follow-up confirmation records of each unit are archived to ensure similar problems won't occur again.
Production Inspection
Our production inspection includes product performance, accuracy, safety and appearance inspection. After the production is inspected, it will be allowed to be packaged. For our production, the products need to be tested before shipping. The purpose of product inspection is to prevent unqualified products from delivering to the customers, which is to avoid customer losses and protect the company's credit.